 |


![]()
 Standard
high-throughput screening (HTS) methods do not allow targeting of specific
sites on a protein. We are screening for both inhibitors and activators
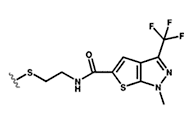
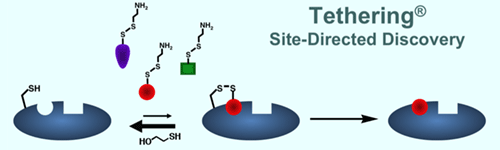
using a novel site-directed chemical biology technology called disulfide
trapping. We are using this approach to determine the roles of specific
inflammatory caspases with selective inhibitors, and to study activation
of kinases in proliferation and apoptosis pathways with allosteric
activators. With this technology, we can trap allosteric states so that
they may be studied by biophysical and mutational means.
Standard
high-throughput screening (HTS) methods do not allow targeting of specific
sites on a protein. We are screening for both inhibitors and activators
using a novel site-directed chemical biology technology called disulfide
trapping. We are using this approach to determine the roles of specific
inflammatory caspases with selective inhibitors, and to study activation
of kinases in proliferation and apoptosis pathways with allosteric
activators. With this technology, we can trap allosteric states so that
they may be studied by biophysical and mutational means.
In collaboration with Dr. Michelle Arkin and her group in the Small Molecule Discovery Center (SMDC), we are also developing high through-put Surface Plasmon Resonance (SPR) technology for fragment screening. These two fragment screening technologies allow us the potential to tackle challenging protein-protein interaction surface targets and allosteric sites. These discovery technologies will help us learn some of the “rules” for how one might more systematically discover compounds that bind these sites. We hope to unlock these challenging target classes for drug discovery.
Wells Lab © 2009 | Site Map